Basicity of amines
Amines, as we know they are compounds obtained by replacing
one or more hydrogen atoms from the ammonia. And we are here to discuss about
basicity of amines.
And we know that amines are basic in nature. And here we are
going to discuss everything about basicity of amines. So let’s get started.
So there are few questions that might interest you
#1. Why amines are basic in nature?
#2. What factors affect basicity of amines?
#3. What is the order of basicity of amines?
And many more other questions occur here in your mind. So
let us discuss them one by one.
Here we are covering following points
- Why Amines are basic in nature?
- Comparison of basicity of amines in various aspects
1. Why amines are basic in nature?
Well the reason behind basicity of amines is quite simple.
We know that amines are compounds containing nitrogen. And nitrogen is third
most electronegative atom in the periodic table. “A species which donates
electrons or accepts proton is a base” according to the Lewis or Lowry
bronsted theory.
So if amines are basic then they must donate electrons or
accept protons, then and then they are basic. Now let’s test that are they
really basic?
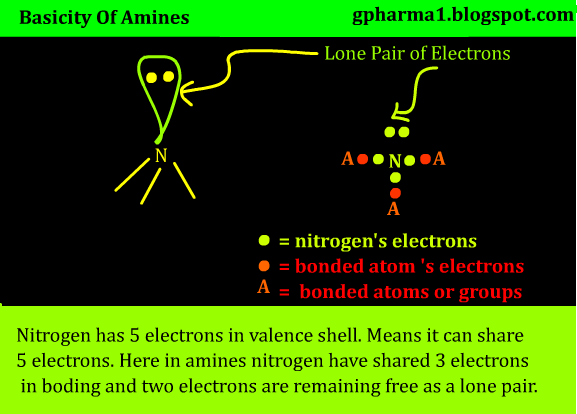
As you can see below, is a fundamental representation of
amines. No matter what groups are attached to it we are focusing on the nitrogen
atom in amines. Nitrogen has 5 electrons in valence shell if you know. Means
nitrogen can share 5 electrons, in covalent bond or co-ordinate bond or any
type of bond.
In this structure nitrogen is attached to 3 groups or atoms (whatever it maybe) via covalent bonds. Means with each atom the nitrogen shared 1 electron in each covalent bond. So nitrogen has shared it’s 3 electrons in bonding. But nitrogen has 5 electrons in its valence shell. So where are remaining two electrons? Yes they are present as a lone pair above nitrogen as shown in structure.
Since nitrogen has lone pair, the electrons in lone pair are
ready to donate by nitrogen. So this means the nitrogen can donate electrons it
in turn means amines can donate electrons. And that proves that amines are
basic in nature according to Lewis acid base theory.
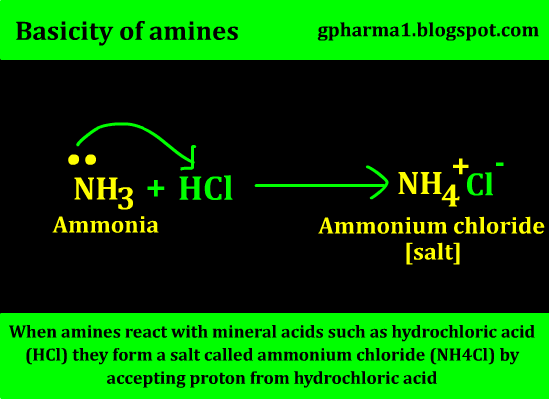
Similarly, when it comes to Lawry bronsted theory, it says that “a species which accepts proton in any reaction is base”. When amines react with mineral acids such as hydrochloric acid (HCl) they form a salt called ammonium chloride (NH4Cl) by accepting proton from hydrochloric acid as shown below.
So again it gets proved that amines are basic in nature according to Lowry bronsted theory.
I hope you got the answer why amines are basic in nature.
2. Comparison of basicity of amines in various aspects
Now let’s compare strength of basicity of amines with other
compounds in short. Amines are aliphatic as well as aromatic.
The more easily the compound or species donates electrons,
more it is basic.
Aromatic amines are less basic than aliphatic amines. The
reason is that there is an inductive effect which helps to increase basicity in
the aliphatic amines, and the electrons in lone pair are totally free to donate
and does not need to go any resonance.
While there is a resonance effect in the aromatic amines
which decreases basicity of amines somewhat. The lone pair in the aromatic
amines is not totally free it takes part in the resonance of the ring,
therefore it cannot donate electrons that much easily that of aliphatic amines.
If we talk about water then amines are much more basic than water, as water is amphoteric in nature.
Amines are less basic than that of hydroxide ions or
ethoxide ions. And they are much more basic than that of alcohols, esters,
ethers etc.
Secondary amines are more basic than that of primary and
tertiary amines. We will discuss this topic in the next part that what is order
of basicity of primary, secondary, and tertiary amines and why secondary amines
are more basic than the primary and tertiary amines.
Hope this helped you little bit. If you have any query or
suggestion comment down below. Have a nice day.






0 Comments