Acidity of phenols:
Today we will
discuss the some theories that are helpful in studying "acidity of phenols".
Let’s get started.
By the Ka values
we can say that phenols are less acidic than carboxylic acid and more acidic
than alcohols.
As we know that acid loses proton in
reaction. Phenol does it too. Phenol losses its hydrogen as a proton and forms
more stable phenoxide ion.
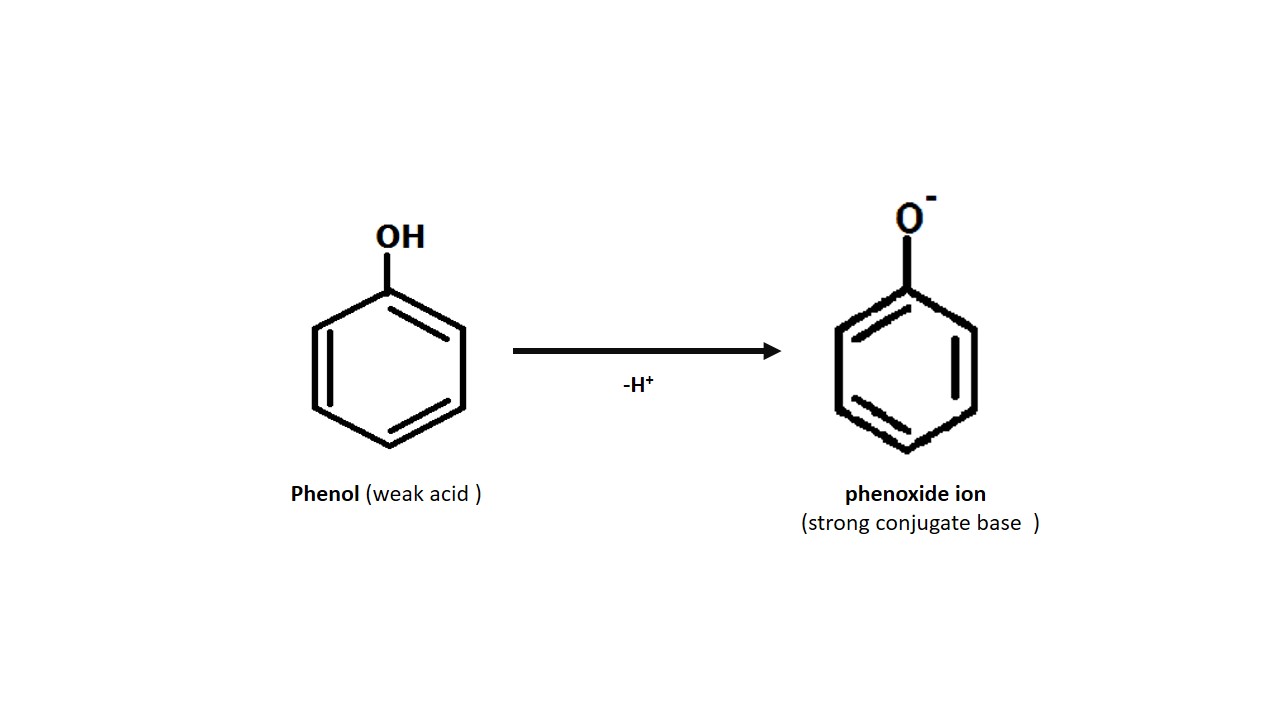 |
As it is more
stable the phenol will always try to lose one proton. Phenoxide ion is stable
because it has many resonating structures stabilising it. Means phenol losses
its proton in a reaction. That confirms that phenol is acidic.
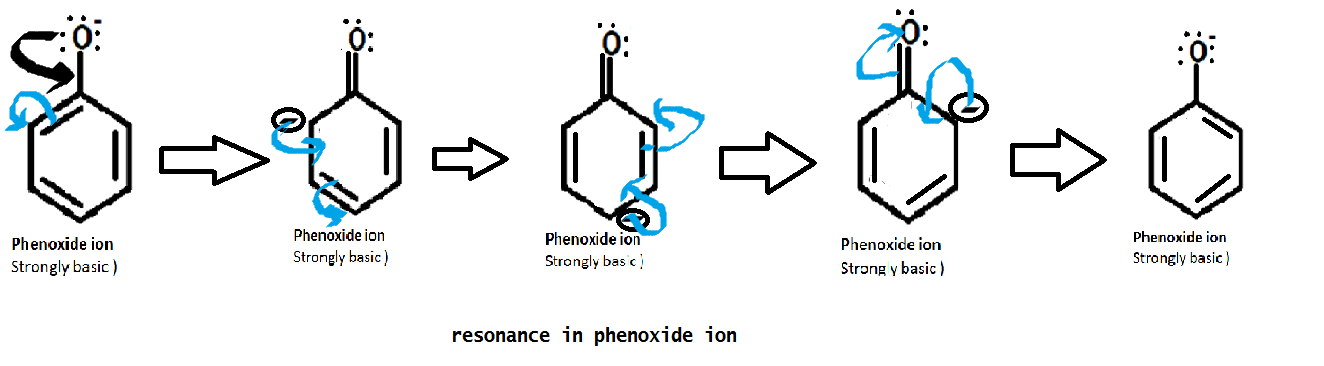
As we
know that phenol losses its proton and forms more stable phenoxide ion. The
formed phenoxide ion is strongly basic (because it has 3 lone pair of electrons
with it and readily donate those electrons).
If u remember conjugate acid base theory that
states that when weak acid loses proton it forms strong conjugate base. Here
phenol losses proton and forms strongly basic conjugate base i.e. phenoxide ion.
That means phenol must be weak acid.
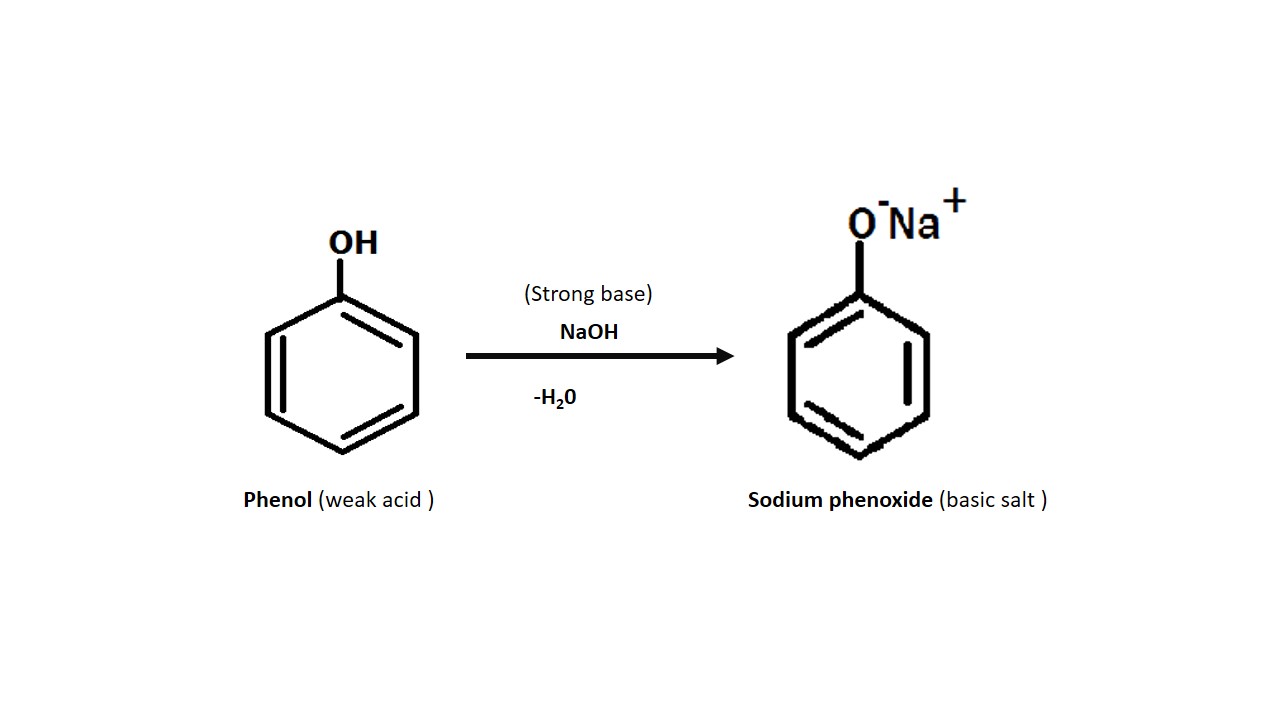
When phenol reacted with the sodium hydroxide then
it forms sodium phenoxide. Which is a basic salt (turns red litmus blue). when
weak acid reacted with stronger base then the salt formed is basic.
Here phenol react with strong base i.e. sodium hydroxide and forms basic salt i.e sodium phenoxide .
Then we can say that phenol is weakly acidic.
Stay tuned for further updates . next blog will come with topic effect of substituents on acidity of phenols .if u like it share & comment .







10 Comments
Excellent work..👍👍
ReplyDeletethnx dear
Delete👍👍
ReplyDeletethnx
DeleteGood work, keep it up , helpful information
ReplyDelete☺thnx
DeleteNice one
ReplyDelete🙏🙏
Deletenice Girish sir
ReplyDelete☺😁thnx
Delete