Effect of substituents on acidity of PHENOLS - detailed explaination:
Substituents have a great effect on acidity and basicity of compounds. Substituents can increase or decrease acidity of phenols. Let’s see how substituents affect acidity of phenols. Let’s get started.
Substituents are of 2 types i.e. Electron withdrawing and Electron donating.
Electron withdrawing groups:
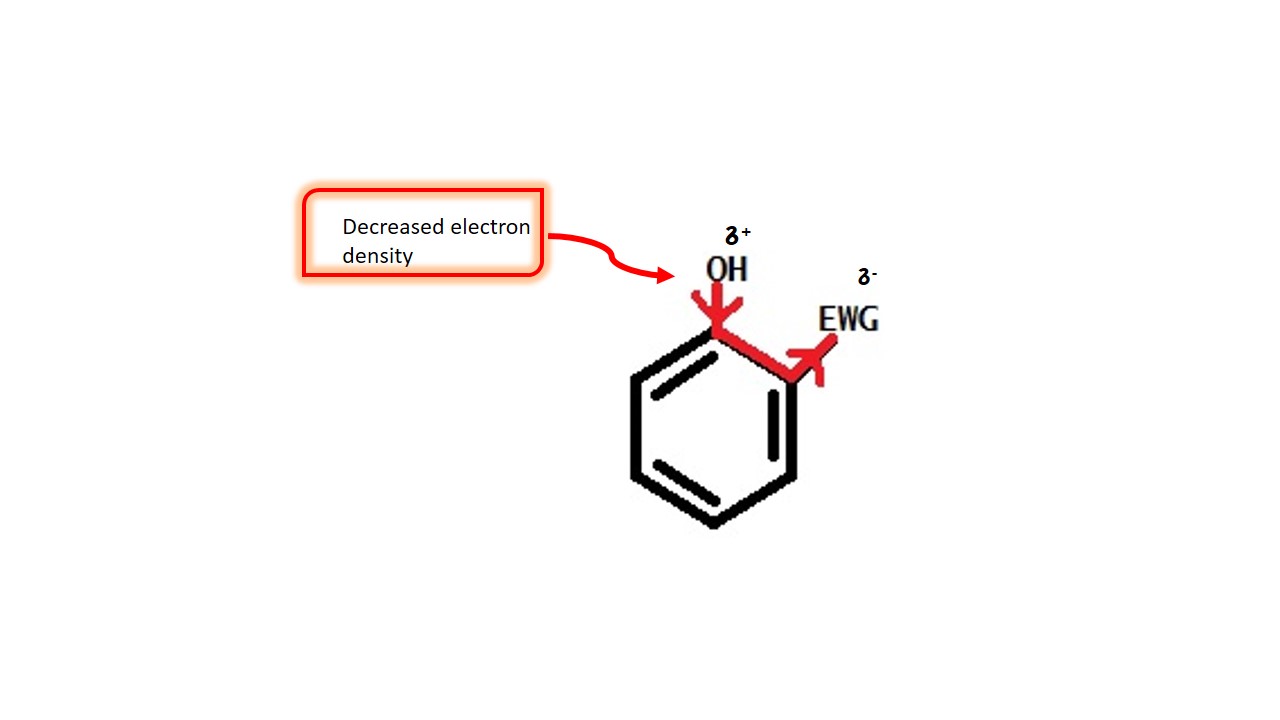
These groups pull electrons from the ring and decrease electron density over the ring.
e.g. –NO2,-COCH3,-CHO, etc. Electron donating groups:
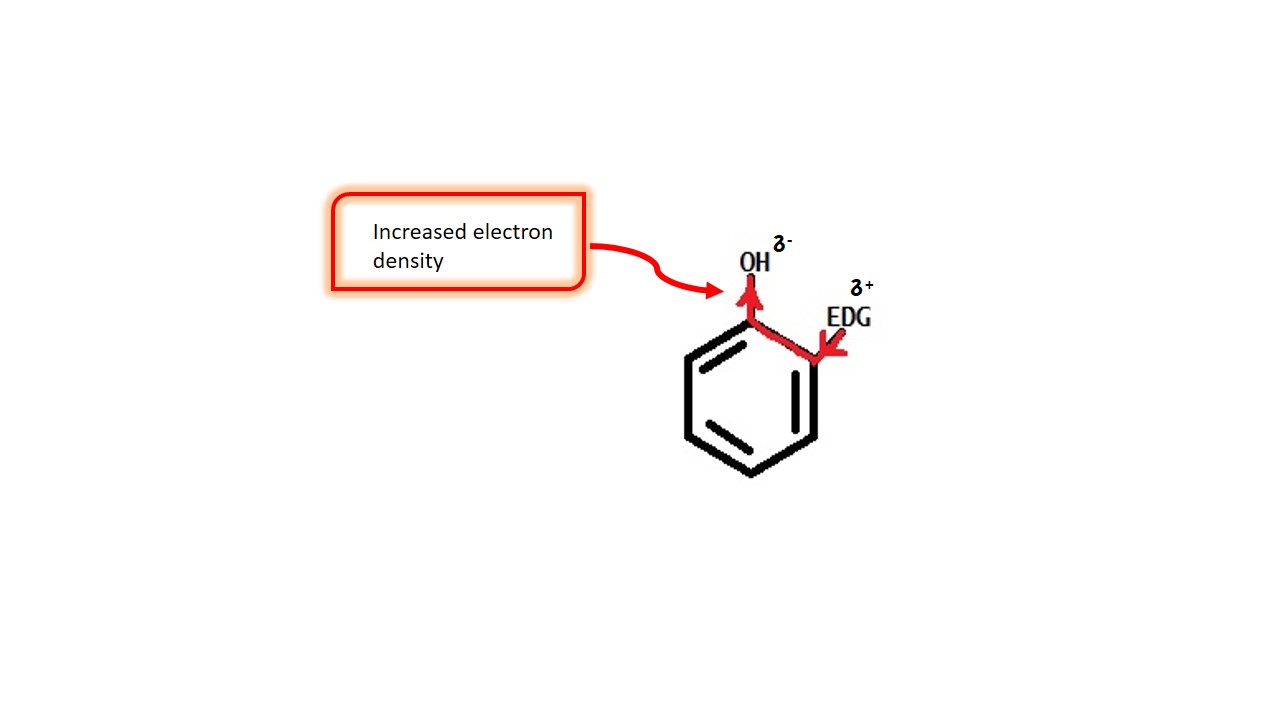
These groups donate electrons to the ring and increase electron density over the ring.e.g. –CH3,-NH2, -OH etc.
Now ,according to Lewis acid base theory, the acid is a species which is able to accept the electron. Now a species will be acidic if it accepts the electron. And to be able to accept electron the species must be electron deficient.
Less the electron density present on the electron accepting group more easily it will accept electron and more acidic it will. High electron density destabilise the structure and make it unable to accept electron and it will less acidic.
So the effect of substituent will depend upon the ability of substituent to increase or decrease the electron density over the structure.
EFFECT OF ELECTRON WITHDRAWING GROUPS:
That makes the phenoxide ion more stable when phenol loses proton. Overall electron withdrawing group helps it to loose proton. That increases acidity of phenol. Collectively we can say that the electron withdrawing groups increase acidity of phenols.
EFFECT OF ELECTRON DONATING GROUPS:
As the name suggest the electron donating groups (such as –NH2,-OH etc.) give electron to the ring and increase electron density over the oxygen in phenol, and destabilise the peroxide ion after releasing proton from phenol.
That makes the phenol difficult to accept electrons and loosing proton. That decreases acidity of phenol. That means electron donating groups decrease acidity of phenols.







3 Comments
very nice content
ReplyDeleteeasy to understand
thank u so much
very nice content
ReplyDeleteeasy to understand
thank u so much
Online Baccarat | Best Baccarat and How to Play in
ReplyDeleteBetting 인카지노 on the game of 바카라 Baccarat 제왕카지노 with an advanced version of our popular online baccarat. Baccarat is a game of chance and luck that offers both ways.