Inductive Effect, Types of Inductive Effect, and Applications of Inductive Effect
Here we will get a basic idea of what is the inductive effect. How it occur? What are the types of Inductive Effect? and What are the applications of Inductive effect?
What is the Inductive effect?
If someone asks us to define the Inductive effect, we can define it as follows.
According to Morrison and Boyd, The inductive effect can be defined as the electron delocalization effect in the chain of a sigma bond of the species where two sigma bonded atoms differ considerably in their electronegativities.
An inductive effect is a permanent effect. Its due to transmission of unequal sharing of the bonding electron through a chain of atoms in a molecule, leading to a permanent dipole in a bond. Inductive effect operates in ground energy state of the molecule.
Let's understand this in a simple way.
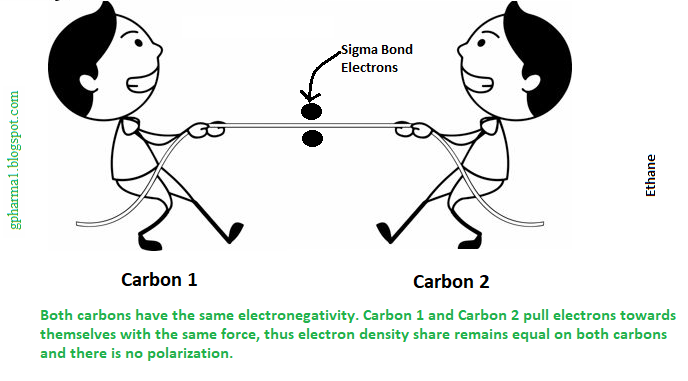
Suppose we have ethane. I consider you all know ethane, an organic compound with 2 carbon atoms and six hydrogen atoms. So below you can see the structure. Here two carbon atoms are joined by a single bond. This single bond is called a sigma bond. Each sigma bond is made up of 2 electrons. As both are carbon atoms, they have the same electronegativity. Thus they pull both electrons with equal force to their side. As forces are the same from both carbons, the electrons of the covalent bond remain at the center.
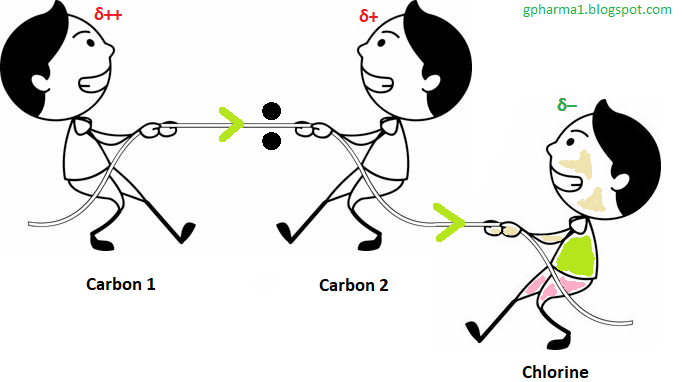
But now we will replace one hydrogen atom of a carbon atom with a chlorine atom. Chlorine is much more electronegative than the carbon atom. So the chlorine atom will pull electrons towards itself from the attached carbon. Due to that, the carbon atom becomes electron deficient (more electronegative) partially due to the effect of the chlorine atom. It needs an electron. So the carbon atom will pull electrons towards itself from the adjacent carbon atom. This means electrons of the covalent bonds will shift towards the chlorine side more.
So this influence is called an Inductive effect.
This is a video from the Youtube channel Khan Academy, just for better understanding and reference for readers.
What are the types of Inductive Effects?
There are two types of inductive effects. +I effect (Positive Inductive Effect) and -I effect (Negative Inductive Effect).
The +I effect (Positive Inductive Effect) is when there is an inductive effect due to the electropositive species. Electropositive species means the species which is electron-rich and can donate electrons. These species are called +I species. And the effect is the +I effect (Positive Inductive Effect).
Various +I species are -CH3, -C2H5, etc.
And -I effect (Negative Inductive Effect) is the exact opposite of that. This means it is caused due to the influence of an electronegative species, which means a species that is able to withdraw electrons. These species are called -I species. And the effect is the -I effect (Negative Inductive Effect).
Various -I species are -Cl,-Br, -CN, -No2, etc.
Difference between +I and -I effect
The table can show you the difference between positive inductive effect and Negative Inductive effects.
| Positive Inductive Effect | Negative Inductive Effect |
|---|---|
| It involves Electron Donating Species | It involves electron withdrawing species |
| In a positive inductive effect, electrons are shifted towards the carbon atom. | In a negative inductive effect, electrons are pulled away from carbon atoms. |
| Positive Inductive Effect is denoted by +I effect | Negative Inductive Effect is denoted by -I effect |
| Various Species involved in the negative inductive effect are -CH3, -C2H5 etc. | Various Species involved in the negative inductive effect are -CH3, -C2H5 etc. |



0 Comments