Hyperconjugation
Various Definitions of Hyperconjugation.
You can always find different definitions of hyperconjugation effect on the internet and books. It's up to you which definition attracts you more. Various Definitions of Hyperconjugation are as follows.
According to Morrison and Boyd, A hyperconjugation effect is a resonance effect where C-H sigma bond snaps and the sigma electron pair from that bond is delocalized on to a C=C bond or empty pi orbital.
The delocalization of σ-electrons or lone pair of electrons into adjacent π-orbital or p-orbital is called hyperconjugation. [egppathshala]
The hyperconjugation effect is a permanent effect in which the localization of σ electrons of the C-H bond of an alkyl group directly attached to an atom of the unsaturated system or to an atom with an unshared p orbital takes place. [Byju's]
Hyperconjugation (σ-conjugation or no-bond resonance) refers to the delocalization of electrons with the participation of bonds of primarily σ-character. Usually, hyperconjugation involves the interaction of the electrons in a sigma (σ) orbital (e.g. C–H or C–C) with an adjacent unpopulated non-bonding p or antibonding σ* or π* orbitals to give a pair of extended molecular orbitals. [Wikipedia]
What is hyperconjugation?
Normally in conjugation, pi electrons delocalize into the orbitals. But in Hyperconjugation, sigma electrons delocalize into the empty pi orbital. Baker and Nathan was first to observe this effect.
A hyperconjugation effect is a resonance effect where C-H sigma bond snaps and the sigma electron pair from that bond is delocalized on to a C=C bond or empty pi orbital.
Hyperconjugation In alkenes
Here we have a propene. The electrons of bond between carbon one and hydrogen get delocalized between carbon 1 and carbon 2 , this causes formation of double bond there. Apparently, the next double bond shifts its electrons to next carbon atom and forms carbanion. The electron deficient Hydrogen atoms remain in molecule.
Hyperconjugation in carbocations
Similarly if we take another example, A carbocation as shown below. The sigma electrons of the C-H bond get delocalized and forms double bond between these two carbons. Where hydrogen gets detached remaining electron deficient in molecule. This is also an example of Hyperconjugation effect.
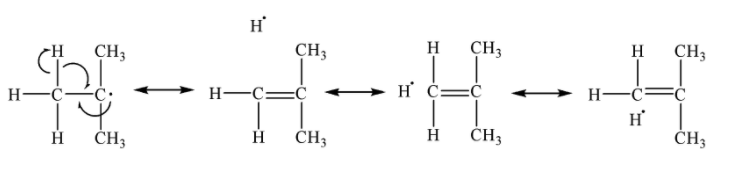
Hyperconjugation in free radicals
Also when it comes to free radicals, Suppose we have a free radical here as shown in figure below. The carbon 2 has a single electron. Here one sigma electron from C-H bond of carbon 1 gets delocalized and forms bond with carbon 2 using its free single electron. This causes formation of double bond. And the Hydrogen remains with 1 electron, in the molecule.
Considering these three examples of Carbanion, Carbocation, Free radical etc. it can be seen that it is type of resonance where there is no bond between C-H while the resonance occurs, Thus it is called the No Bond Resonance.
Now lets see this at molecular orbital level. Lets take example number 1 from above and see what happened in molecular orbitals. There is double bond between carbon 2 and carbon 3. The Pi orbitals of both of them have single single electrons. And carbon 1 have 2 sigma electrons between C-H bond.
.png) |
| Source: EGPPathshala |
Now in hyperconjugation, orbitals of carbon 1 and carbon 2 overlap , and one electron from the carbon 1 orbital delocalizes to the pi orbital of carbon 2. Then the existing pi electron in carbon 2 orbital move to the pi orbital of carbon 3 and it causes formation of lone pair over carbon 3 and thus carbanion. While there is formation of double bond between carbon 1 and carbon 2. The detached hydrogen remains electron deficient and remain in the molecule.
Requirements For Hyperconjugation
Following are the requirements for Hyperconjugation effect.
Presence of α-CH group / lone pair on atom adjacent to sp2 hybrid carbon (Carbon with Double Bond) or other hetero atoms like nitrogen, oxygen etc.
Applications of Hyperconjugation Effect:
- Hyperconjugation effect plays important role in the
- Stability of alkenes
- Stability of carbocations
- Stability of free radicals
- Dipole moment and Bond Length
- Rate and regioselectivity of Electrophilic Substitution of alkylbenzenes
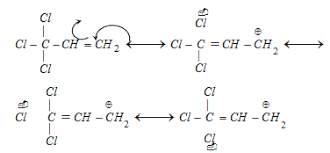
What is reverse Hyperconjugation?
Assignment
- In Hyperconjugation which types of electrons are involved? Sigma or Pi?
- Is hyperconjugation a permanent effect or temperory one?
- Why Hyperconjugation effect is called as No Bond Resonance?
- Why Hyperconjugation effect is called as ?
- What are the various applications of Hyperconjugation effect?
- Who discovered Hyperconjugation Effect?
- What are the requirements for the Hyperconjugation effect?
- What is Reverse Conjugation?


.webp)




0 Comments